A research paper authored by Shibata Laboratory published in an ACS journal was selected as an "ACS Editors' Choice" article.
Category:News|Publishing : August 1, 2022
A research paper titled "Synthesis of Pyridine-SF4-Isoxazolines Using the Functionality of trans-Tetrafluoro-λ6-sulfanyl Rodlike Linkers," authored by Koki Maruno (then, Life Science and Applied Chemistry Program, Department of Engineering, 2nd year of Master's Course), Kenshiro Hada (Life Science and Applied Chemistry Program, Department of Engineering, 1st year of Master's Course) et al. at Shibata Laboratory published in Organic Letters, a scientific journal published by the American Chemical Society (ACS), was highly evaluated by Editors and selected as an "ACS Editors' Choice" article. ACS Editors' Choice is a program in which world-renowned editors select one outstanding article per day from among 64 or more peer-reviewed journals published by ACS. Articles selected as ACS Editors' Choice articles are free to read for a limited period of time, and ACS members and subscribers of ACS's journals are notified accordingly by email. Also, each selected article bears a logo (Fig. 1) indicating that it has become an ACS Editors' Choice article (Fig. 2).
(Figure1) (Figure2)
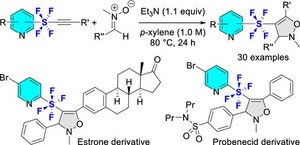
The following is a description of this highly evaluated research paper. The tetrafluoro-λ6-sulfanyl (SF4) moiety has distinctive properties, including high fat solubility, strong electron-withdrawing effect, and ability to build linear geometry. However, this has not been adequately studied. In this study, Shibata Laboratory has successfully synthesized pyridine−SF4−isoxazolines, in which the two heterocycles are linearly connected by a SF4 linker, via the regioselective 1,3-dipolar cycloaddition of pyridine−SF4−alkynes and nitrones. The SF4 moiety is expected to be used as an alternative to para-substituted benzenes, alkynes and bicyclo[1.1.1] pentyl derivatives, which have linear geometry, and is also effective in drug design. Pyridine−SF4−isoxazolines, which were synthesized in this study, are expected to be applied to medical and agrochemical products (Fig. 3).
(Figure3)
<Journal containing this article>
Journal name: Organic Letters
Title: Synthesis of Pyridine-SF4-Isoxazolines Using the Functionality of trans-Tetrafluoro-λ6-sulfanyl Rodlike Linkers
Authors: Koki Maruno, Kenshiro Hada, Yuji Sumii, Osamu Nagata, Norio Shibata
Article information: DOI: 10.1021/acs.orglett.2c01046
Website containing the published article
RESEARCH NEWS - Turning Fish Waste into Quality Carbon-based Nanomaterial Nagoya Institute of Technology and Toyota Industries Corporation launch joint research on smart plants and warehouses

 Japanese
Japanese