The research accomplishment of Ms. Shino Inukai(second year of the Master's Course), who is a member of the Kandori and Katayama Lab , was chosen as the Cover Art for Journal of Biological Chemistry
Category:News|Publishing : September 7, 2023
The study, Investigating the Mechanism of Photoisomerization in Jellyfish Rhodopsin with the Counterion at an Atypical Position, was published in Journal of Biological Chemistry, and chosen as its Cover Art for the July issue. Authors of the study were Shino Inukai (second year of the Master's course), Kota Katayama, PhD, Hideki Kandori, PhD, in the Department of Life Science and Applied Chemistry, Nagoya Institute of Technology, and Akihisa Terakita, PhD, Mitsumasa Koyanagi, PhD, in the Department of Biology, Osaka Metropolitan University.
Animal rhodopsins are light-sensitive proteins that typically functions as G protein-coupled receptors (GPCRs). Many animals use animal rhodopins as light sensors for various physiological functions, such as vision and nonvisual functions. More than several thousands of rhodopsins have been identified from a wide variety of animals, which possess multiple rhodopsin genes. Furthermore, the diversification of these rhodopsins translates into a diversification of the types of G proteins coupling (Gt, Gi, Go, and Gq). A possible molecular mechanism for the evolution of animal rhodopsins has been the counterion displacement theory. The counterion is a key residue in animal rhodopsins that maintain sensitivity to visual light and facilitate the photoisomerization cycle. It has only been located at two positions in animal rhodopsins, position 113 in the transmembrane helix 3 (TM3) in vertebrate rhodopsins and position 181 in the 2nd extracellular loop (ECL2) in invertebrate rhodopsins. The evolutionary displacement of the counterion into the TM region is associated with increased efficiency of G protein activation. Recent research has found that the box jellyfish, an invertebrate, rhodopsin (JelRh) utilizes a different amino acid as counterion, which is located at TM2. The counterion of JelRh is positioned similarly to that of vertebrate rhodopsin, suggesting that the two evolved in a convergent manner. In addition, JelRh is the first animal rhodopsin known to activate Gs protein. Its ability to control the Gs signaling pathway with light makes it a promising new optogenetics tool for regulating repeatedly cAMP induction, which is widely associated with biological event such as circadian rhythms, cardiac function, and behavioral control. However, the molecular mechanism behind how JelRh's photoreaction properties are affected by its novel counterion positioning and whether it is related to its ability to activate Gs protein is still unknown.
The research team presented the structural change in the retinal binding pocket of JelRh by measuring low temperature UV-visible and FTIR spectroscopies, and compared the similarities and differences in the retinal structure, isomerization process, and protein interaction caused by the displacement of a counterion in TM2 when compared to both vertebrate (bovine rhodopsin: BovRh) and invertebrate rhodopsin (squid rhodopsin: SquRh). The team found that the manner of retinal isomerization occurring upon photoabsorption and the retinal structure of JelRh are similar to those of BovRh, rather than SquRh. In addition, the strength of the interaction between the retinal Schiff base and the counterion was also almost identical to that of BovRh. On the other hand, JelRh exhibited a unique hydrogen bonding network, constituted by amino acids and water molecules around the retinal chromophore, indicative of a hybrid behavior combining both BovRh-like and SquRh-like structural changes. Thus, this study revealed the first structural element of JelRh, which is the only animal rhodopsin with a counterion in TM2 and an active Gs protein.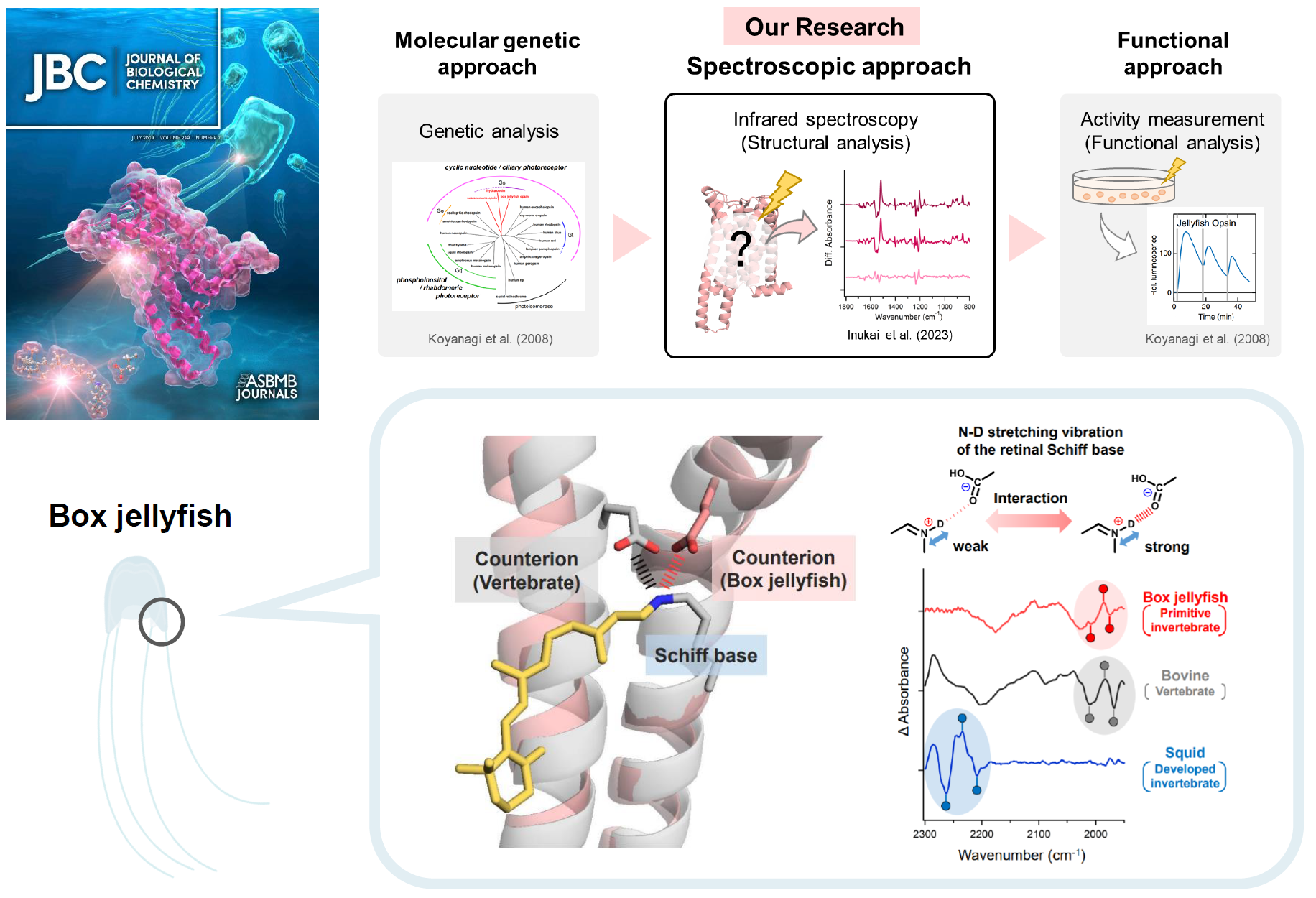
Table of Contents page: Journal of Biological Chemistry (jbc.org)
Research Paper by Professor Shibata and Team Graces Organic Letters with Impressive "Supplementary Cover Art" RESEARCH NEWS - Ceramic Tea Set Glazing Affects Health Benefits of Tea, Finds New Study

 Japanese
Japanese