RESEARCH NEWS Nagoya Institute of Technology Researchers Propose Novel BaTiO3-Based Catalyst for Oxidative Coupling of Methane
Category:News|Publishing : November 19, 2025
Perovskites--a class of compounds with a unique ABX3 structure and high temperature stability--are promising materials for energy conversion. In recent years, they have been utilized in photovoltaic systems. They exhibit excellent performance in solid oxide fuel cells and organic reactions such as oxidative coupling of methane (OCM) for the production of ethane and ethylene.
Notably, BaTiO3 is a promising perovskite with applications in fields including ferroelectricity, piezoelectricity, and semiconductivity. It possesses a flexible lattice and rich defect chemistry, making it suitable for structural modifications via doping for enhanced functional performance. Furthermore, it offers benefits such as high temperature resistance, cost-effectiveness, and mature industrial-scale production technology.
In a recent breakthrough, a team of researchers led by Mr. GAN Rongguang, a PhD student from Nagoya Institute of Technology (NITech), along with Dr. NISHIDA Yoshihide and Prof. Dr. HAYAKAWA Tomokatsu from NITech, Japan, and Mr. ZIEGLER Andreas and Prof. Dr. MEYER Bernd from Friedrich-Alexander-Universitat Erlangen-Nürnberg, Germany, has successfully modified BaTiO3 with 3 wt% Ca for OCM. Their study was made available online on July 26, 2025, and was published in Volume 713 of the journal Applied Surface Science on December 15, 2025.
"I was intrigued by how surface structures of oxides often change during catalytic reactions. Understanding such dynamic behavior could help interpret experimental results correctly, and that curiosity gradually developed into this study," says Mr. GAN.
The team innovatively combined experimental observation, via field-emission scanning electron microscopy, transmission electron microscopy, X-ray photoelectron spectroscopy, and oxygen and carbon dioxide temperature-programmed desorption, with density functional theory-based theoretical analysis to demonstrate that surface reconstruction of BaTiO3 using Ca leads to the formation of characteristic TiO-like structural motifs, which facilitates the generation of reactive oxygen species on the surface. The emergence of these species is likely linked to methane activation in terms of reaction product selectivity. In this way, the local motifs influence the oxidative methane-coupling properties of the oxide surface.
"The Ti2+ state on BaTiO3 materials possesses a different redox power than the standard Ti3+ state. Our research by demonstrating how to create and stabilize this specific Ti2+ motif, provides scientists with a new tuning knob to precisely tailor a material's surface to perform one specific chemical job exceptionally well, such as achieving high C2 selectivity," says Mr. GAN.
The knowledge gained from this study can serve as a basis for improving the stability and reactivity of oxide materials used in catalysis and energy conversion not only from a fundamental viewpoint but also for industrial development.
"In the future, the proposed principle could be used for developing smart catalysts for many industrial processes, such as production of everyday plastics, medicines, and fuels, leading to reduced energy consumption and fewer unwanted by-products," concludes Mr. GAN.
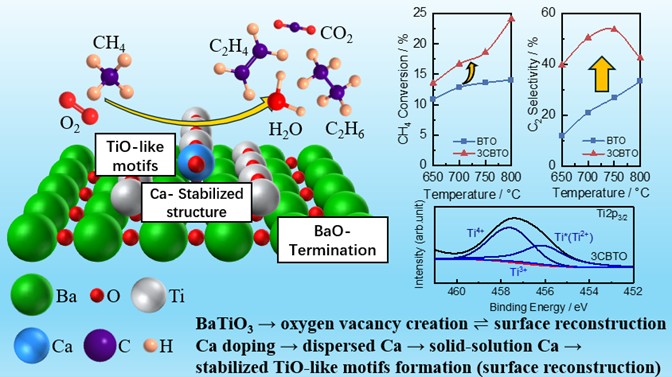
Researchers demonstrate that BaTiO3 modified with 3 wt% Ca leads to unique reduced structural motifs on the surface, significantly enhancing the catalytic performance of oxidative coupling of methane in terms of reaction product selectivity.
Reference
|
Title of original paper |
Effective surface modifications on BaTiO3 : linking structural motifs to methane coupling performance |
|
Journal |
Applied Surface Science |
|
DOI |
10.1016/j.apsusc.2025.164164 |
|
Latest Article Publication Date |
15 December 2025 |
Funding information
The study received financial support from the Japan Society for the Promotion of Science (JSPS) Japanese-German Graduate Externship (Grant No. 2019/R1) and the Deutsche Forschungsgemeinschaft (DFG) through Graduate School GRK 2495/L (project number 399073171). R. G. was self-funded.
Contact
Prof. HAYAKAWA Tomokatsu
Ph.D. Supervisor of Rongguang Gan
E-mail : hayakawa.tomokatsu[at]nitech.ac.jp
ORCID: 0000-0003-1817-8854
Mr. GAN Rongguang
E-mail: r.gan.311[at]nitech.jp
ORICD: 0009-0003-2492-6166
*Please replace [at] with @ when contacting.
Academic Exchange Agreements concluded with IITGN (India) RESEARCH NEWS Gas-Switch Reduction Enables Alloying in Supported Catalysts

 Japanese
Japanese